Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (PRACTICE SHEET (ADVANCED))|24 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE 1 (LONG ANSWER QUESTIONS)|7 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - I (MAIN))|13 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II (PRACTICE SHEET ADVANCE)) (Integer Type Questions)|3 VideosELEMENTS OF CARBON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (Recent AIPMT/NEET Questions) |10 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELEMENTS OF BORON FAMILY-ADDITIONAL PRACTICE EXERCISE (LEVEL - II LECTURE SHEET (ADVANCED))
- Electronic structure acquired by compounds of IIIA group elements in b...
Text Solution
|
- Thallous chlorie is more stable than thallic chloride because of
Text Solution
|
- Element with gaint co - valent structure is
Text Solution
|
- Which of the following is used in high temperature thermometry ?
Text Solution
|
- The maximum co - valancy of aluminium is '6' where as that of boron is...
Text Solution
|
- Which element reacts with acids as well as alkalies.
Text Solution
|
- Which statements are correct. (1) I.P of 'Ga' is greater than 'Al'...
Text Solution
|
- The electrical conductivity of aluminium is twice that of copper on th...
Text Solution
|
- 'Al' and its alloy can be given shapes of
Text Solution
|
- Which of the following minerals contain aluminium ?
Text Solution
|
- Aluminium becomes passive in
Text Solution
|
- The chief impurity not present in bauxite is
Text Solution
|
- AlCl(3) is an electron deficient compound but AlF(3) is due to
Text Solution
|
- Which of the following element donot form carbide
Text Solution
|
- Al(2)(SO(4))(3)+NH(4)OH rarr X,X is
Text Solution
|
- An inorganic compound (A) shows the following reactions. It is white s...
Text Solution
|
- An inorganic compound (A) shows the following reactions. It is white s...
Text Solution
|
- {:(,"Column - I",,"Column - II"),((A),"Corundum",(P),Ca(a)B(6)O(11).5H...
Text Solution
|
- How many of the following show borax bead test B, Be, Mg, Cu, Fe, Cr, ...
Text Solution
|
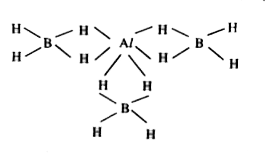
- Al[BH(4)](3) contains how many 3 centered -2e^(-) bonds.
Text Solution
|