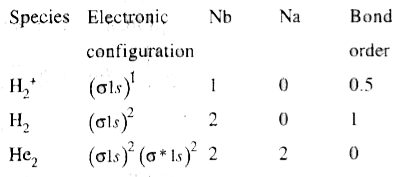Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE - 1 (LONG ANSWER QUESTIONS)|5 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE - 1 (SHORT ANSWER QUESTIONS)|3 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|20 VideosCHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|30 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL BONDING-OBJECTIVE EXERCISE -3 (RECENT AIPMT/NEET QUESTIONS )
- Write the electronic configurations and calculate the bond orders of H...
Text Solution
|
- In which of the following pairs, the two species are isostructural?
Text Solution
|
- The correct order of increasing bond angles in the following triatomic...
Text Solution
|
- In which of the following molecules/ions BF(3), NO(2),NH(2) and H(2)O ...
Text Solution
|
- Four diatomic species are listed below in different sequencies. Which ...
Text Solution
|
- According to MO theory which of the lists rank the nitrogen species in...
Text Solution
|
- What is the dominant intermolecular force or bond that must be overcom...
Text Solution
|
- In which of the following pairs of molecules/ ions, the central atoms ...
Text Solution
|
- Which one of the folllowing species does not exist under normal condit...
Text Solution
|
- In which one of the following species the central atom has the type of...
Text Solution
|
- Some of the properties of the two species, NO(2)^(-) and H(3)O^(+) are...
Text Solution
|
- In which of the following molecules the central atom does not have sp^...
Text Solution
|
- Which of the following has the minimum bond length?
Text Solution
|
- Which of the two ions from the list given below that have the geometry...
Text Solution
|
- The correct order of increasing bond length of C-H, C-O, and C=C is
Text Solution
|
- The pairs of species of oxygen and their magnetic behaviour are noted ...
Text Solution
|
- Bond order of 1.5 is shown by
Text Solution
|
- Which of the following species contains thre bond pairs and one Ione p...
Text Solution
|
- The pair of species with the same bond order is
Text Solution
|
- During change of O(2) to O(2)^(-) ion, the electron adds on which one ...
Text Solution
|
- Four diatomic species are listed below. Identify the correct order in ...
Text Solution
|