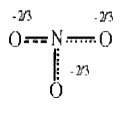
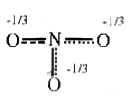
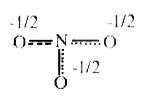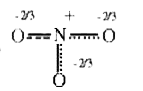A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE-2B|82 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE-3|48 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE-2A (DIPOLE MOMENT)|6 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|20 VideosCHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|30 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL BONDING-OBJECTIVE EXERCISE-2A (BOND CHARACTERS)
- Which of the following has least bond energy?
Text Solution
|
- Which of the following hydrocabon has least C-C bond length?
Text Solution
|
- The decreasing order of bond dissociation energies of C-C,C-H and H -H...
Text Solution
|
- Which of the following has largest bond angle?
Text Solution
|
- The resonance hybrid of nitrate ion is
Text Solution
|