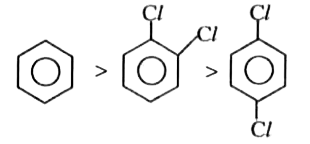
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
AAKASH SERIES|Exercise EXERCISE ON PASSAGE|16 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE -1 (Long answer questions)|5 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE-2B|82 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|20 VideosCHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|30 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL BONDING-OBJECTIVE EXERCISE-3
- Which bond angle theta would result in the maximum dipolemoment for th...
Text Solution
|
- If the bond length and dipolemoment of a diatomic molecule are 1.25A^@...
Text Solution
|
- (A): The dipole moment value of NH3 is greater than zero (B): In NH3 b...
Text Solution
|
- (A): SiF4 is non polar even though fluorine is much more electronegat...
Text Solution
|
- A: SO(2) molecule has unsymmetrical shape R: The dipole moment of SO...
Text Solution
|
- The following are some statements about dipole moment. i. The dipole...
Text Solution
|
- From the following given statements of the order of dipolemoments. (...
Text Solution
|
- Which of the following has highest dipole moment?
Text Solution
|
- The Cl-O bond order in perchlorate ion
Text Solution
|
- Which of the following is more stable
Text Solution
|
- The bond dissociation of the molecules A2 , B2,C2 are 498, 158, 945 kJ...
Text Solution
|
- The table shown lists the bond dissociation energies (E("diss")) for s...
Text Solution
|
- The correct order of N -O bond length in NO, NO(2)^(-) , NO(3)^(-) an...
Text Solution
|
- The correct order of increasing C-O bond length of CO,CO(3)^(2-) and C...
Text Solution
|
- The number and type of bonds between two carbon atoms in CaC(2) are
Text Solution
|
- KF combines with HF to form KHF(2). The compound contains the species
Text Solution
|
- The bond order of individual carbon bonds in benzene is
Text Solution
|
- In which of the following species the inter atomic bond angle is 109^@...
Text Solution
|
- Which one of the following pairs of molecules will have permenant dipo...
Text Solution
|
- Which of the following compounds has the smallest bond angle in its mo...
Text Solution
|