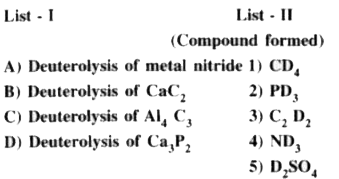Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROGEN AND ITS COMPOUNDS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 2B|60 VideosHYDROGEN AND ITS COMPOUNDS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3|48 VideosHYDROGEN AND ITS COMPOUNDS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 2A (WATER, HARD WATER, HEAVY WATER)|20 VideosHYDROCARBONS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE SHEET ( LEVEL-II (PRACTICE SHEET (ADVANCED) (MATRIX MATCHING TYPE QUESTIONS)))|5 VideosIONIC EQUILIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))|8 Videos
AAKASH SERIES-HYDROGEN AND ITS COMPOUNDS-OBJECTIVE EXERCISE - 2A (HYDROGEN PEROXIDE)
- H(2) gas is liberated when H(2)O(2) reacts with
Text Solution
|
- The reagent used in the conversion: C6H6 to C6H5 - OH is
Text Solution
|
- H(2)O(2) cannot act as
Text Solution
|
- H(2)O(2) reduces
Text Solution
|
- The following reaction shows the reducing action of H(2)O(2)
Text Solution
|
- Solid H2O2 has non planar and non linear structure based on
Text Solution
|
- The volume strength of 1.5 N H2O2 is
Text Solution
|
- Catalytic union of H2 and O2 to get H2O2 is found in
Text Solution
|
- W/V percentage of 1 M H2O2 solution is
Text Solution
|
- Volume strength of perhydrol is
Text Solution
|
- The molarity of 5.6V H2O2 is
Text Solution
|
- The normality of 2.24 vol H2O2 is
Text Solution
|
- H2O2 forms prismatic crystal at
Text Solution
|
- The weight of iodine liberated when excess of KI reacts with 500 ml of...
Text Solution
|
- The orange coloured compound formed when H2O2 is added TiO2 lon acidif...
Text Solution
|
- When H(2)O(2) is treated with a solution of titanium dioxide in conc. ...
Text Solution
|
Text Solution
|
Text Solution
|
Text Solution
|
Text Solution
|