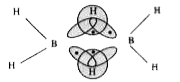
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The number of 3 centered, 2 electron bonds in doborane is
Text Solution
|
- If the electron pair forming a bond between two atoms and B is not in ...
Text Solution
|
- The number of 2-centre-2-electron and 3-centre-2-electron bonds in B(2...
Text Solution
|
- तीन केंद्र दो इलेक्ट्रॉन बंध पाये जाता हैं :
Text Solution
|
- दो इलेक्ट्रॉन तीन केंद्रीय बंध (two electron three centre bond) किसे क...
Text Solution
|
- Steric Repulsion | Multi Centered Bonds | 3 centered 2 electron Bond
Text Solution
|
- B2H6 में 2-केन्द्र-2-इलेक्ट्रॉन तथा 3-केन्द्र-2-इलेक्ट्रॉन आबन्धों की ...
Text Solution
|
- Diborane is simplest and most familiar hydride of boton. Its chemical ...
Text Solution
|
- Diborane is simplest and most familiar hydride of boton. Its chemical ...
Text Solution
|