A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Boric acid B(OH)(3) is weak monobasic acid reacts with alkali to form...
Text Solution
|
- Boron form many compound like boric acid, borax inorganc graphite etc....
Text Solution
|
- Boron form many compound like boric acid, borax inorganc graphite etc....
Text Solution
|
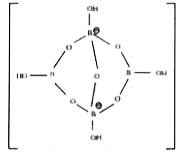
- Borax is actually made of two tetrahedral and tetrahedral and two tria...
Text Solution
|
- Borax is actually made of two tetrahedral and tetrahedral and two tria...
Text Solution
|
- बोरेक्स बनता है जब बोरिक ऐसिड निम्न के साथ क्रिया करता है -
Text Solution
|
- बोरेक्स से बोरिक अम्ल
Text Solution
|
- बोरिक अम्ल से ट्राइमिथाइल बोरेट
Text Solution
|
- बोरेक्स तथा बोरिक अम्ल का सूत्र लिखिए।
Text Solution
|