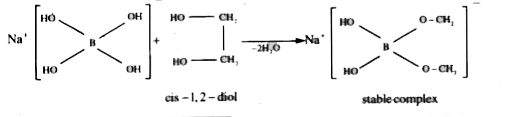
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- B(OH)(3)+NaOH hArr NaBO(2)+Na[B(OH)(4)]+H(2)O How can this reactio...
Text Solution
|
- How can the following reaction be made to proceed in forward direction...
Text Solution
|
- B(OH)(3)+NaOH rarr Na[B(OH)(4)]+H(2)O How can this reaction is made ...
Text Solution
|
- B(OH)(3)+NaOH to Na[B(OH)(4)] (aq) . Then addition of which of the fol...
Text Solution
|
- 2B(OH)(3) + 2NaOHhArr NaBO(2) + Na[B(OH)(4)]+2H(2)O How can this re...
Text Solution
|
- B(OH)(3)+NaOH square square square NaBO(2) +Na[B(OH)(4)]+H(2)O How c...
Text Solution
|
- B(OH)(3)+NaOH square square square NaBO(2) +Na[B(OH)(4)]+H(2)O How c...
Text Solution
|
- B(OH)3+NaOHhArrNa[B(OH)4] How this reaction can is made to proceed in ...
Text Solution
|
- B(OH)(2) +NaOH hArr BaBO (2) + Na [ B (OH)(4) ] + H (2) O यह अभिक्र...
Text Solution
|