A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Recommended Questions
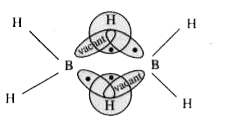
- Diborane is simplest and most familiar hydride of boton. Its chemical ...
Text Solution
|
- The type of hybridisation of boron in diborane is (a) sp , (b) sp^(...
Text Solution
|
- डाइबोरेन में बोरान का संकरण कौन सा होता है - (क) sp (ख) sp^2 ...
Text Solution
|
- The type of hybridisation of boron is diborane is (a) sp , (b) sp^(2) ...
Text Solution
|
- Assertion : In diborane, each B atom is sp^(2) hybridised. Reason : In...
Text Solution
|
- A : In Diborane containing eight -B-H bonds only four B-H bonds are on...
Text Solution
|
- Assertion : In diborane containing eight -B-H bonds, the four terminal...
Text Solution
|
- Assertion : In diborane containing eight -B-H bonds, the four terminal...
Text Solution
|
- The type of hybridisation of boron in diborane is (a) sp , (b) sp^(2) ...
Text Solution
|