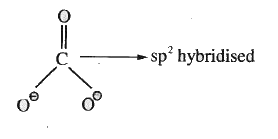
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELEMENTS OF CARBON FAMILY
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II PRACTICE SHEET INTEGER TYPE QUESTIONS)|5 VideosELEMENTS OF CARBON FAMILY
AAKASH SERIES|Exercise ADDITIONAL QUESTIONS|10 VideosELEMENTS OF CARBON FAMILY
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II PRACTICE SHEET MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)|5 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3|7 VideosENVIRONMENTAL CHEMISTRY
AAKASH SERIES|Exercise EXERCISE ON PASSAGE (PASSAGE-III)|5 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELEMENTS OF CARBON FAMILY-ADDITIONAL PRACTICE EXERCISE (LEVEL - II PRACTICE SHEET LINKED COMPREHENSION TYPE QUESTIONS)
- Tin contains only 5 - 10 % of tin as SnO(2), the rest being Siliceous ...
Text Solution
|
- Tin contains only 5 - 10 % of tin as SnO(2), the rest being Siliceous ...
Text Solution
|
- Tin contains only 5 - 10 % of tin as SnO(2), the rest being Siliceous ...
Text Solution
|
- Carbon shows allotropy. The various allotropic forms of carbon can be ...
Text Solution
|
- Carbon shows allotropy. The various allotropic forms of carbon can be ...
Text Solution
|