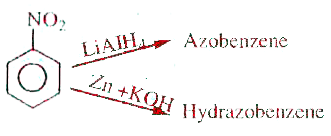A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AMINES AND AZO COMPOUNDS
AAKASH SERIES|Exercise LEVEL - I (EXERCISE-II) (AMINES GENERAL)|5 VideosAMINES AND AZO COMPOUNDS
AAKASH SERIES|Exercise LEVEL - I (EXERCISE-II) (ANILINE)|5 VideosAMINES AND AZO COMPOUNDS
AAKASH SERIES|Exercise LEVEL - I (EXERCISE-I) DIAZONIUM SALTS (Properties-applications:)|6 VideosALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 4 (ASSERTION (A) & REASON (R) TYPE QUESTIONS)|19 VideosAMINES AND DIAZONIUM SLATS
AAKASH SERIES|Exercise PRACTICE EXERCISE|25 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-AMINES AND AZO COMPOUNDS -LEVEL - I (EXERCISE-II)
- Nitrobenzene to Hydrazobenzene. Here the reagent is
Text Solution
|
- In the reaction The equivalent weight of Nitrobenzene is
Text Solution
|
- R-overset(O)overset(||)N to O and R-O-N = O are a pair of
Text Solution
|
- Here the reagent is
Text Solution
|
- The ratio of the number of hydrogen atoms required to get 1 mole of az...
Text Solution
|
- the main product of the reaction
Text Solution
|
- Which of the following staements is wrong i) Shape of a aliphatie am...
Text Solution
|