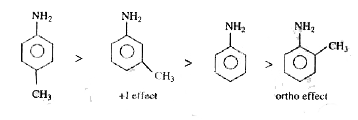A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AMINES AND AZO COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (More than one correct answer questions)|5 VideosAMINES AND AZO COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (Linked Comprehension type questions) Passage - I|3 VideosAMINES AND AZO COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 1 (Matching Type Questions)|9 VideosALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 4 (ASSERTION (A) & REASON (R) TYPE QUESTIONS)|19 VideosAMINES AND DIAZONIUM SLATS
AAKASH SERIES|Exercise PRACTICE EXERCISE|25 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-AMINES AND AZO COMPOUNDS -PRACTICE SHEET - 2 (Single answer questions)
- The product of the reaction is
Text Solution
|
- Which of the following statements regarding is true
Text Solution
|
- The Structure of the product is
Text Solution
|
- Consider the following (II) Order of Basic nature is more...
Text Solution
|
- The structure of the product B is
Text Solution
|
- Arrange p-methylaniline (I), m-methyl aniline (II), aniline (III), o-m...
Text Solution
|
- Which of the following is the strongest Bronsted acid ?
Text Solution
|
- In which of the following reactions give only primary amine
Text Solution
|
- Which of the following sequence orf reactions is useful to prepare com...
Text Solution
|
- The structure of B is
Text Solution
|