A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REVISION EXERCISE
AAKASH SERIES|Exercise SURFACE CHEMISTRY|45 VideosREVISION EXERCISE
AAKASH SERIES|Exercise VA GROUP ELEMENTS|26 VideosREVISION EXERCISE
AAKASH SERIES|Exercise CHEMICAL KINETICS|39 VideosREDOX REACTONS
AAKASH SERIES|Exercise Questions For Descriptive Answers|27 VideosSOLID STATE
AAKASH SERIES|Exercise PRACTICE SHEET - 5|30 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-REVISION EXERCISE -ELECTROCHEMISTRY
- The conductivity of saturated solution of AgCl is found to be 1.86 xx ...
Text Solution
|
- At 25^@ C, the ionic mobility of CH3COO^(-), H^+ are respectively 4.1 ...
Text Solution
|
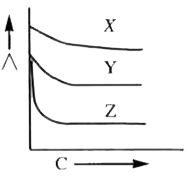
- Equivalent conductance (Lamda) vs concentration graphs are given for s...
Text Solution
|
- For the redox reaction : Zn(s) + Cu^(2+) (0.1M) to Zn^(2+) (1M) + ...
Text Solution
|
- E(Cu^(2+)|Cu)^@ = +0.337 V, E(Zn^(2+)| Zn)^@ = - 0.762 V. The EMF of...
Text Solution
|
- For a cell reaction involving a two-electron change, the standard e.m....
Text Solution
|
- E(Sn^(2+)|Sn)^@ = -1.14V, E(Pb^(2+)IPb)^@ = 0.126 The cell reaction of...
Text Solution
|
- The EMF of the following cells are Cu|Cu^(2+) (1M)||Ag^(+) (1M)|Ag...
Text Solution
|
- Given the data at 25^@C Ag + I^(-) to AgI + e^(-) , E^@ = 0.152V ...
Text Solution
|
- The potential of the cell containing two hydrogen electrodes as repres...
Text Solution
|
- For the following cell Zn//Zn^(2+) // Cd^(2+) // Cd E("cell") = ...
Text Solution
|
- The standard reduction potentials for two reactions are given below: ...
Text Solution
|
- What is the approximate quantity of electricity in 500 ml 1M AgNO3 aq...
Text Solution
|
- The cell , Zn//Zn^(2+) (1M) // Cu^(2+) (1M) // Cu (E("Cell")^@ = 1.10...
Text Solution
|
- If the number of moles of electrons involved in a reaction is doubled ...
Text Solution
|
- The standard EMF of Daniel cell is 1.10 volt. The maximum electrical w...
Text Solution
|
- If the Pb^(2+) concentration is maintained at 1.0M what is the [Cu^(2+...
Text Solution
|
- The standard e.m.f. for the cell reaction, 2Cu^(+)(aq) to Cu(s) + ...
Text Solution
|
- The standard e.m.f. of a galvanic cell involving cell reaction with n ...
Text Solution
|
- Standard electrode potentials of Fe^(2+) + 2e to Fe and Fe^(3+) + 3...
Text Solution
|