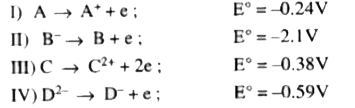A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REVISION EXERCISE
AAKASH SERIES|Exercise SURFACE CHEMISTRY|45 VideosREVISION EXERCISE
AAKASH SERIES|Exercise VA GROUP ELEMENTS|26 VideosREVISION EXERCISE
AAKASH SERIES|Exercise CHEMICAL KINETICS|39 VideosREDOX REACTONS
AAKASH SERIES|Exercise Questions For Descriptive Answers|27 VideosSOLID STATE
AAKASH SERIES|Exercise PRACTICE SHEET - 5|30 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-REVISION EXERCISE -ELECTROCHEMISTRY
- On passing 3F electricity through three cells containing fused Na2CO3 ...
Text Solution
|
- For the electrolytic production of NaClO4 from NaClO3 according to t...
Text Solution
|
- The quantity of electricity in Faradays required to reduce 1.23 gm of ...
Text Solution
|
- When 3.86 amperes current are passed through an electrolyte for 50 min...
Text Solution
|
- When 965 amp current is passed through aqueous solution of salt X usin...
Text Solution
|
- What is the approximate quantity of electricity (in coulomb) required ...
Text Solution
|
- Four moles of electrons were transferred from anode to cathode in an e...
Text Solution
|
- One Faraday of electricity is passed separately through one litre of o...
Text Solution
|
- The resistance of 0.5 N solution of an electrolyte in a conductivity c...
Text Solution
|
- 0.05M NaOH solution offered a resistance of 31.69Omega in a conducti...
Text Solution
|
- The resistance of 0.05M KCl solution at 25^@C is 100 ohm in a cell wh...
Text Solution
|
- Aluminium displaces hydrogen from acids, but copper does not. A galvan...
Text Solution
|
- When E(Ag^(+) | Ag)^@ = 0.80 volt and E(Zn^(2+)|Zn)^@ = 0.76 volt, wh...
Text Solution
|
- Deduce from the following E^@ values of half cells, what combination o...
Text Solution
|
- The half cell reaction for the corrosion are , 2H^(+) + (1)/(2) O2 + ...
Text Solution
|
- A cell constructed by coupling a standard copper electrode and a stand...
Text Solution
|
- E^@ values of Zn^(2+), Zn and Cu^(2+), Cu are respectively -0.76 V a...
Text Solution
|
- E^@ of Fe|Fe^(2+) is +0.44V , E^@ of Cu|Cu^(2+) is -0.32V. Then in the...
Text Solution
|
- The standard reduction potentials of Ag, Cu, Co and Zn are 0.799, 0.33...
Text Solution
|
- The standard reduction potentials of Zn^(2+)//Zn,Cu^(2+)//Cu and Ag^(+...
Text Solution
|