Similar Questions
Explore conceptually related problems
Recommended Questions
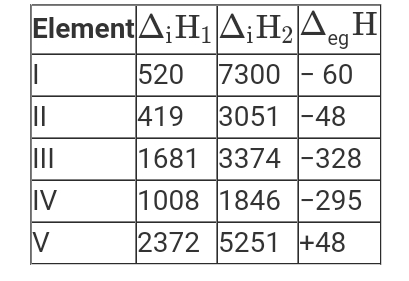
- The first (Delta(i)H(1)) and second (Delta(i)H(2)) ionization enthalpi...
Text Solution
|
- The first (Delta(i)H(1)) and second (Delta(i)H(2)) ionisation enthalpi...
Text Solution
|
- The second ionization enthalpies (Delta(i)H(2)) of alkali metals are
Text Solution
|
- The first (DeltaIH1) and second (DeltaIH2) ionisation enthalpies (in k...
Text Solution
|
- The first ionization enthalpy (Delta(i)H) values of the second period ...
Text Solution
|
- The first (Delta(i)H(1) and second (Delta(i)H(2) ionization enthalpies...
Text Solution
|
- The first (Delta(i)H(1) and second (Delta(i)H(2) ionization enthalpies...
Text Solution
|
- कुछ तत्वों की प्रथम Delta(1)H(1) और द्वितीय Delta(1) H(2) आयनन एन्थैल्...
Text Solution
|
- The first (Delta(i)H(1)) and the second (Delta(i)H(2)) ionization enth...
Text Solution
|