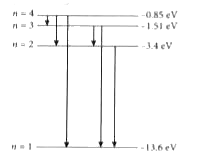
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG GUIDE-ATOMS AND NUCLEI-AIPMT/NEET(MCQs)
- Ratio of longest wavelengths corresponding to Lyman and Balmer series ...
Text Solution
|
- A certain mass of hydrogen is changes to helium by the process of fusi...
Text Solution
|
- The half-life of a radioactive isotope X is 20 years. It decays to ano...
Text Solution
|
- Hydrogen atom in ground state is excited by a monochromatic radiation ...
Text Solution
|
- The binding energy per nucleon of .(3)^(7) Li and .(2)^(4)He nuclei ar...
Text Solution
|
- A radio isotope X with a half-life 1.4 xx 10^9 years decays of Y which...
Text Solution
|
- If radius of the .(13)^(27)Al nucleus is taken to be R(AI), then the r...
Text Solution
|
- Consider 3rd orbit of He^(+) (Helium) using nonrelativistic approach t...
Text Solution
|
- A nucleus of uranium decays at rest into nuclei of thorium and helium....
Text Solution
|
- In the spectrum of hydrogen atom, the ratio of the longest wavelength ...
Text Solution
|
- Given the value of Rydberg constant is 10^(7)m^(-1), the waves number ...
Text Solution
|
- When an alpha-particle of mass 'm' moving with velocity 'v' bombards o...
Text Solution
|
- If an electron in a hydrogen atom jumps from the 3rd orbit to the 2nd ...
Text Solution
|
- The half-life of a radioactive substance is 30 minutes, The time (in m...
Text Solution
|
- Radioactive material 'A' has decay constant '8 lamda' and material 'B'...
Text Solution
|
- The ratio of wavelength of the lest line of Balmer series and the last...
Text Solution
|
- For a radioactive material, half-life is 10 minutes. If initially ther...
Text Solution
|
- The ratio of kinetic energy to the total energy of an electron in a Bo...
Text Solution
|
- The total energy of an electron in an atom in an orbit is -3.4eV. Its...
Text Solution
|
- alpha-particle consists of
Text Solution
|