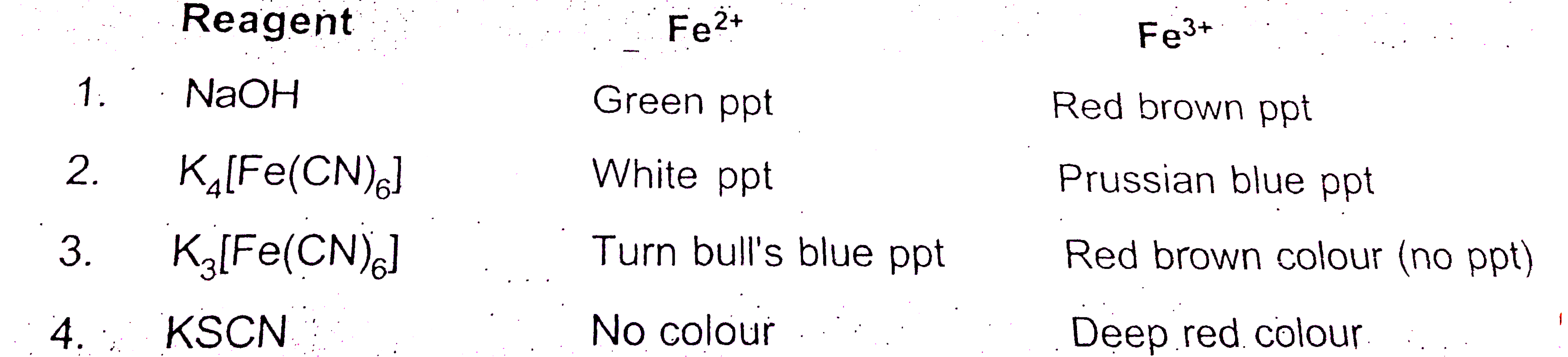Text Solution
Verified by Experts
Topper's Solved these Questions
THE D AND F-BLOCK ELEMENTS
AAKASH INSTITUTE|Exercise Try Yourself|15 VideosTHE D AND F-BLOCK ELEMENTS
AAKASH INSTITUTE|Exercise Assingnment(Section -A ( Objective Type Questions)(One option is correct))|50 VideosTest-12
AAKASH INSTITUTE|Exercise Exercise|30 VideosTHE P-BLOCK ELEMENTS
AAKASH INSTITUTE|Exercise Assignment Section-J)|16 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-THE D AND F-BLOCK ELEMENTS -Assingment ( Section-J Aakash Challengers Questions )
- Given chemical test for making distinction between Fe^(2+) and Fe^(3+...
Text Solution
|
- KMnO(4) dissollution in concentration H(2)SO(4) results in explosion d...
Text Solution
|
- When K(2)MnO(4) is added in solution of NH(4)Cl then
Text Solution
|
- Which of the following lanthanoids has highest tendency to form comple...
Text Solution
|
- What will the structure of CrO(5) in presence of pyridine ?
Text Solution
|
- Choose the correct statement regarding bonding in FeCl(3) (I) It c...
Text Solution
|
- The hybridisation of Cu in (NH(4))(2)[CuCl(4)] and Cs(2)[CuCl(4)] is
Text Solution
|
- Stability of the complex may depend on
Text Solution
|
- In this diagram , the most stable oxidation is
Text Solution
|
- The correct regarding CuCl(5)^(-3) compound is
Text Solution
|
- What will be the hybridisation of Ni(CN)(5)^(-3) ?
Text Solution
|