Similar Questions
Explore conceptually related problems
Recommended Questions
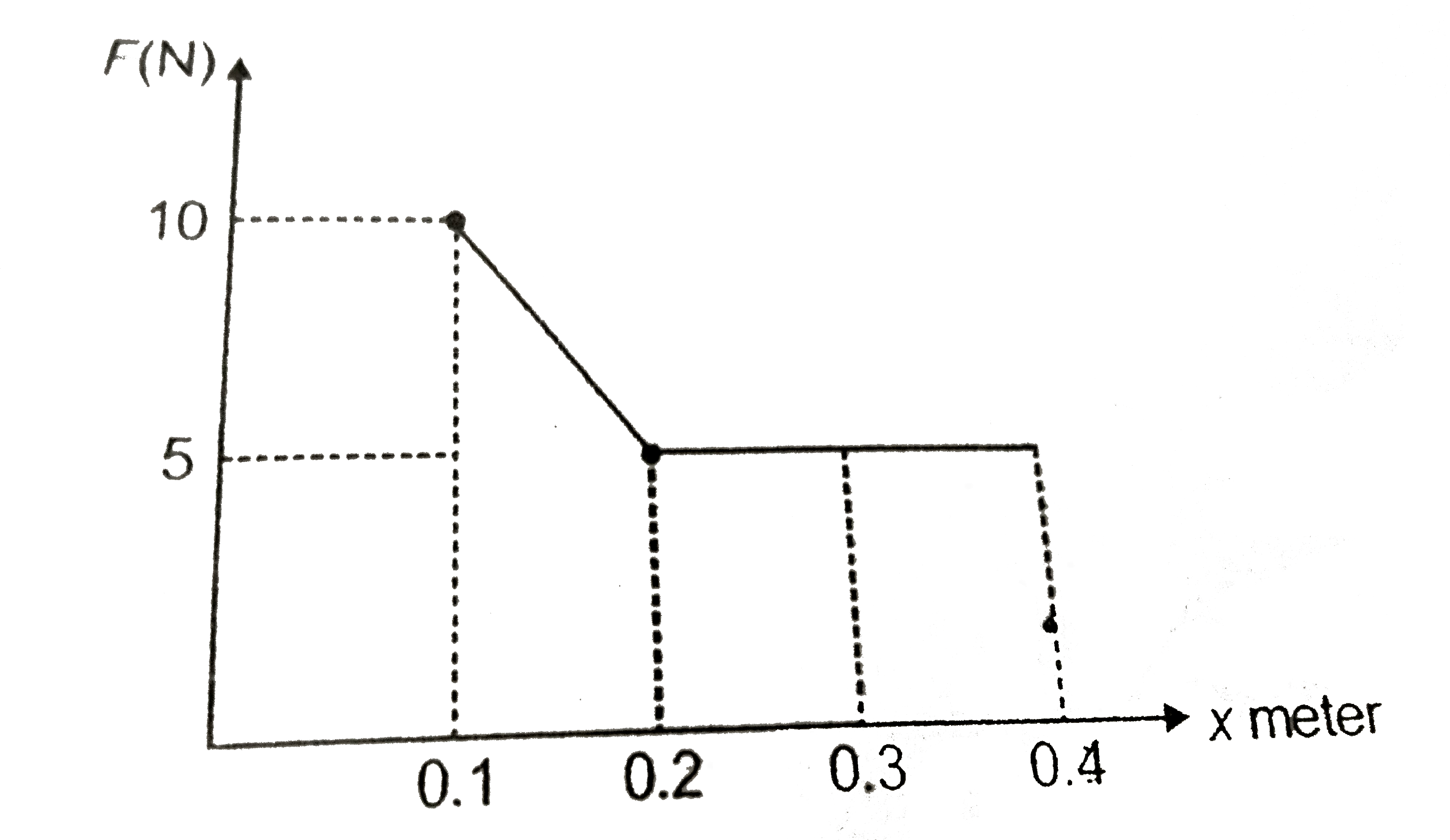
- The given figrue shows the variation of force exerted on the piston by...
Text Solution
|
- Shows a process ABCA performed on an ideal gas. Find the net heat give...
Text Solution
|
- The given figure shows the variation of force applied by ideal gas on ...
Text Solution
|
- A vertical hollow cylinder contains an ideal gas. The gas is enclosed ...
Text Solution
|
- When an ideal gas in a cylinder was compreswsed isothermally by a pist...
Text Solution
|
- The given figrue shows the variation of force exerted on the piston by...
Text Solution
|
- PV Diagram for ideal gas in piston cylinder assembly undergoing a ther...
Text Solution
|
- When the gas enclosed beneath the piston shown in the figure receives ...
Text Solution
|
- Figure Shows a process ABCA performed on an ideal gas. Find the net he...
Text Solution
|