Similar Questions
Explore conceptually related problems
Recommended Questions
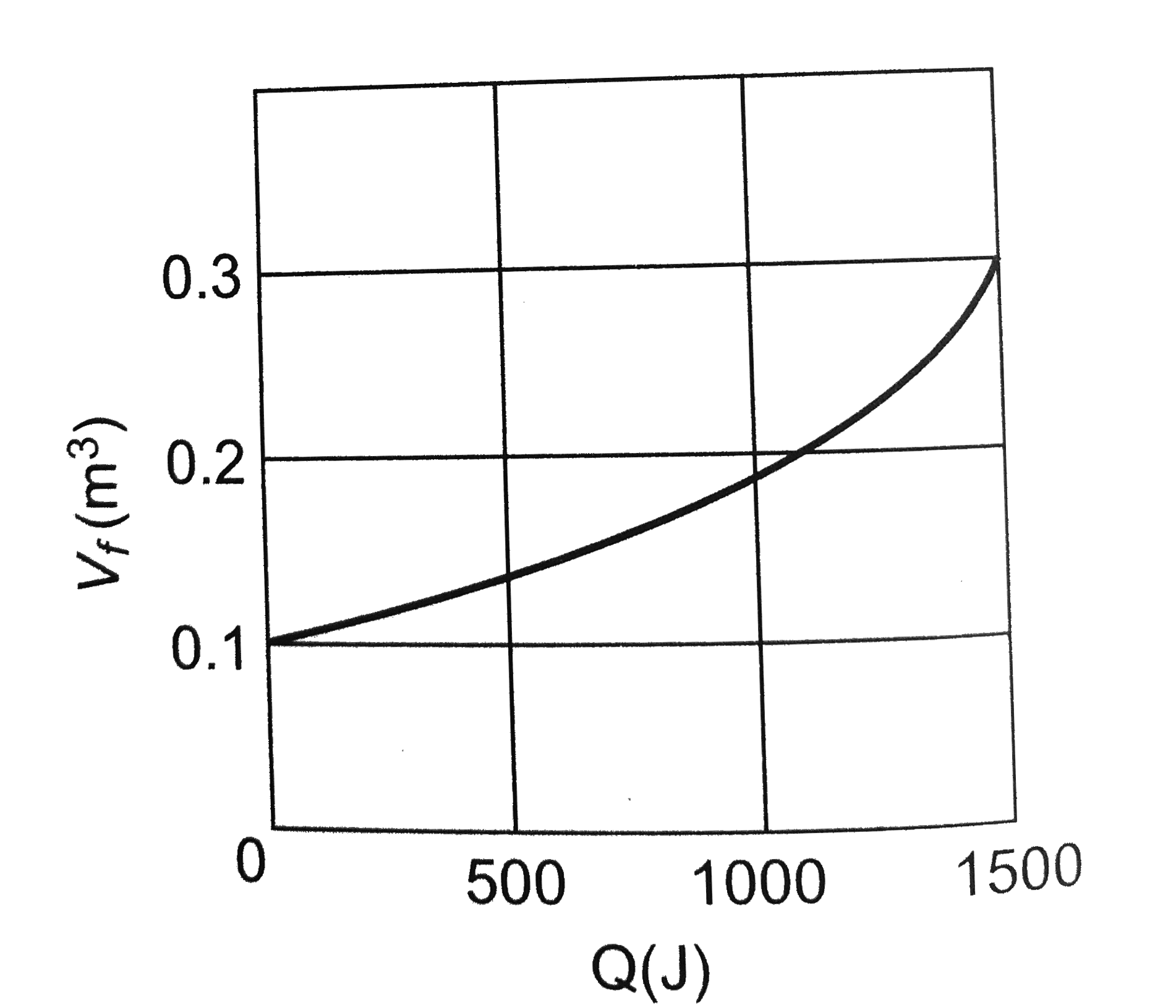
- Suppose 0.5 moles of an ideal gas undergoes an isothermal expansion as...
Text Solution
|
- Suppose 0.5 moles of an ideal gas undergoes an isothermal expansion as...
Text Solution
|
- One mole of an ideal gas at 27^(@) ,8.21 atm absorbs 420 cal of heat d...
Text Solution
|
- Consider isothermal expansion of 0.5 mole of an ideal gas when Q amoun...
Text Solution
|
- Entrolpy change in isothermal expansion work of one mole of an ideal g...
Text Solution
|
- Entropy change for an isothermal expansion of one mole of an ideal gas...
Text Solution
|
- Suppose 0.825 mol of an ideal gas undergoes an isothermal expansion as...
Text Solution
|
- 0.5 mole of gas at temperature 300 K expands isothermally from an init...
Text Solution
|
- 0.5 mole of gas at temperature 300 K expands isothermally from an init...
Text Solution
|