Similar Questions
Explore conceptually related problems
Recommended Questions
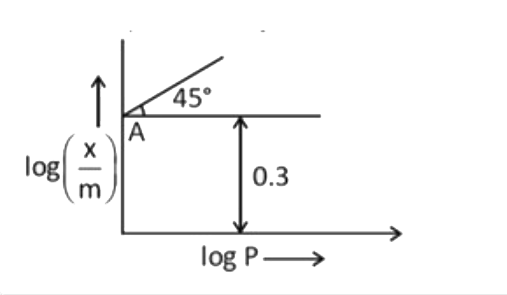
- Graph between log((x)/(m)) vs log P is provided for adsorption of NH(3...
Text Solution
|
- When a graph is plotted between log x//m and log p, it is striaght lin...
Text Solution
|
- From the following graph of log x//m us log P, calculate mass of H(@) ...
Text Solution
|
- Graph between log x/m and log P is a straight line at angle of 45^(@) ...
Text Solution
|
- When a graph is pplotted between log x//m and log p, it is straight li...
Text Solution
|
- Graph between log((x)/(m)) vs log P is provided for adsorption of NH(3...
Text Solution
|
- Graph between log((x)/(m)) and log P is straight line at angle of 45^(...
Text Solution
|
- एक गैस का अधिशोषण, फ्रैण्डलिक अधिशोषण समताप का पालन करता है। अधिशोषक ...
Text Solution
|
- एक अधिशोषण प्रयोग में, log (x/m) व log P के मध्य ग्राफ 45% ढाल के साथ ...
Text Solution
|