Similar Questions
Explore conceptually related problems
Recommended Questions
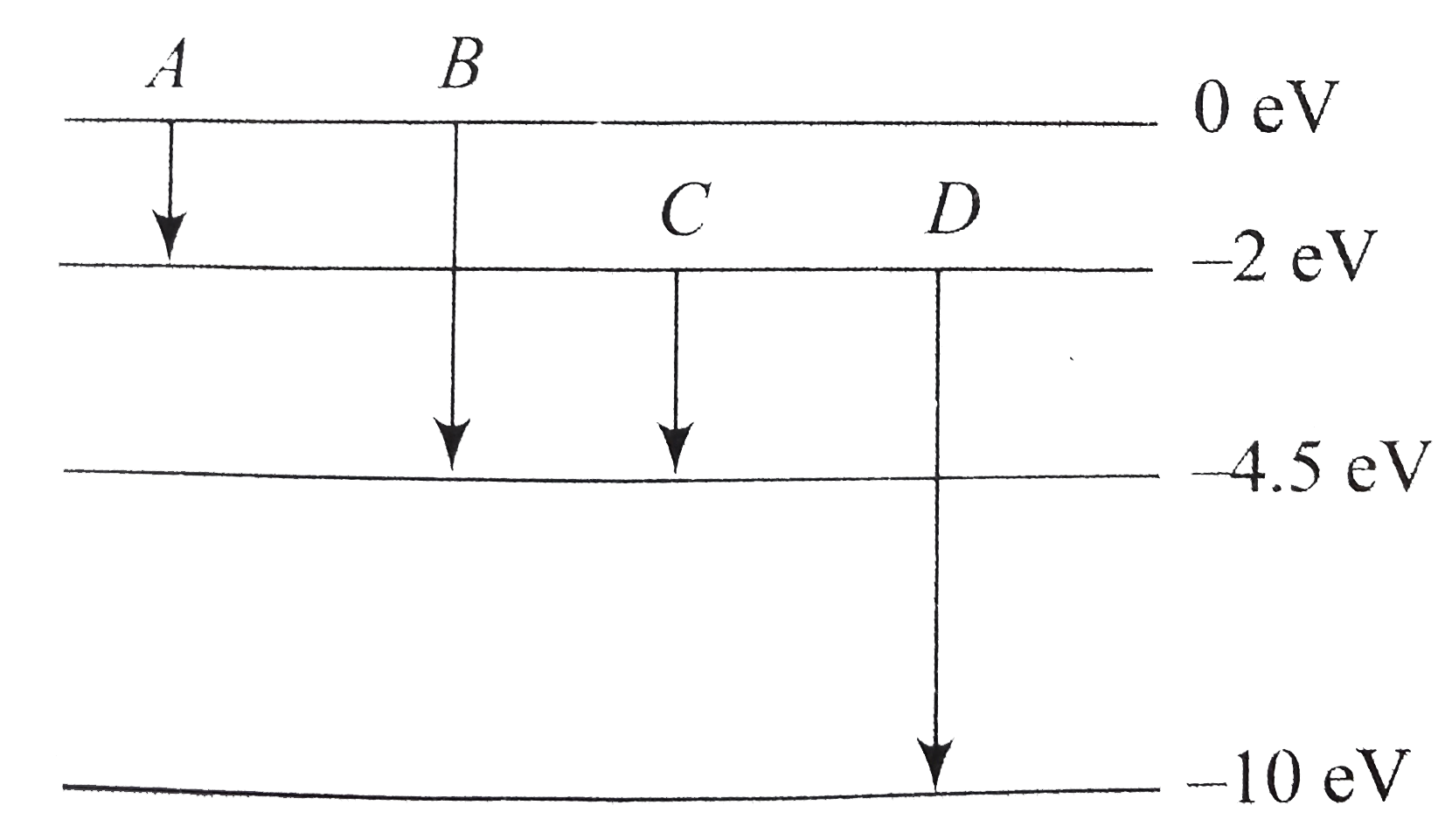
- The energy levels of an atom are as shown in figure . Which one of tho...
Text Solution
|
- The disgram shown the energy levels for an electron is a certain atom ...
Text Solution
|
- The energy levels of an atom are as shown in figure . Which one of tho...
Text Solution
|
- The diagram shown the energy levels for an electron in a certain atom....
Text Solution
|
- Fig. shows energy level diagram of hydrogen atom. Find out the transit...
Text Solution
|
- Shows the energy levels for an electron in a certain atom. Which trans...
Text Solution
|
- The energy levels of an atom of element X are shown in Fig. 20.2. Whic...
Text Solution
|
- The energy levels of a hypothetical aton are shown below. Which of the...
Text Solution
|
- एक परमाणु के ऊर्जा स्तर नीचे दिए चित्र में प्रदर्शित हैं । प्रदर्शित उ...
Text Solution
|