Similar Questions
Explore conceptually related problems
Recommended Questions
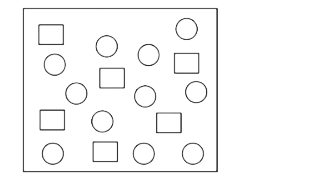
- In the figure shown below reactant A (represented by square) is in equ...
Text Solution
|
- For a system in equilibrium, the rate constant for the forward reactio...
Text Solution
|
- Reactant A represented by square is in equilibrium with product B repr...
Text Solution
|
- Assertion : The equilibrium constant for the reverse reaction is equal...
Text Solution
|
- In the figure shown below reactant A (represented by square) is in equ...
Text Solution
|
- For reactions involving gaseous reactants and products the equilibrium...
Text Solution
|
- When reactants and products are in gaseous state, the equilibrium cons...
Text Solution
|
- इनमें में से कौनसी अभिक्रिया में, साम्यवस्था पर उत्पाद की सांद्रता, अभ...
Text Solution
|
- When reactants and products are in gaseous state, the equilibrium cons...
Text Solution
|