Similar Questions
Explore conceptually related problems
Recommended Questions
- 1//R(R"is universal gas constant") moles of an ideal gas (gamma=1.5) u...
Text Solution
|
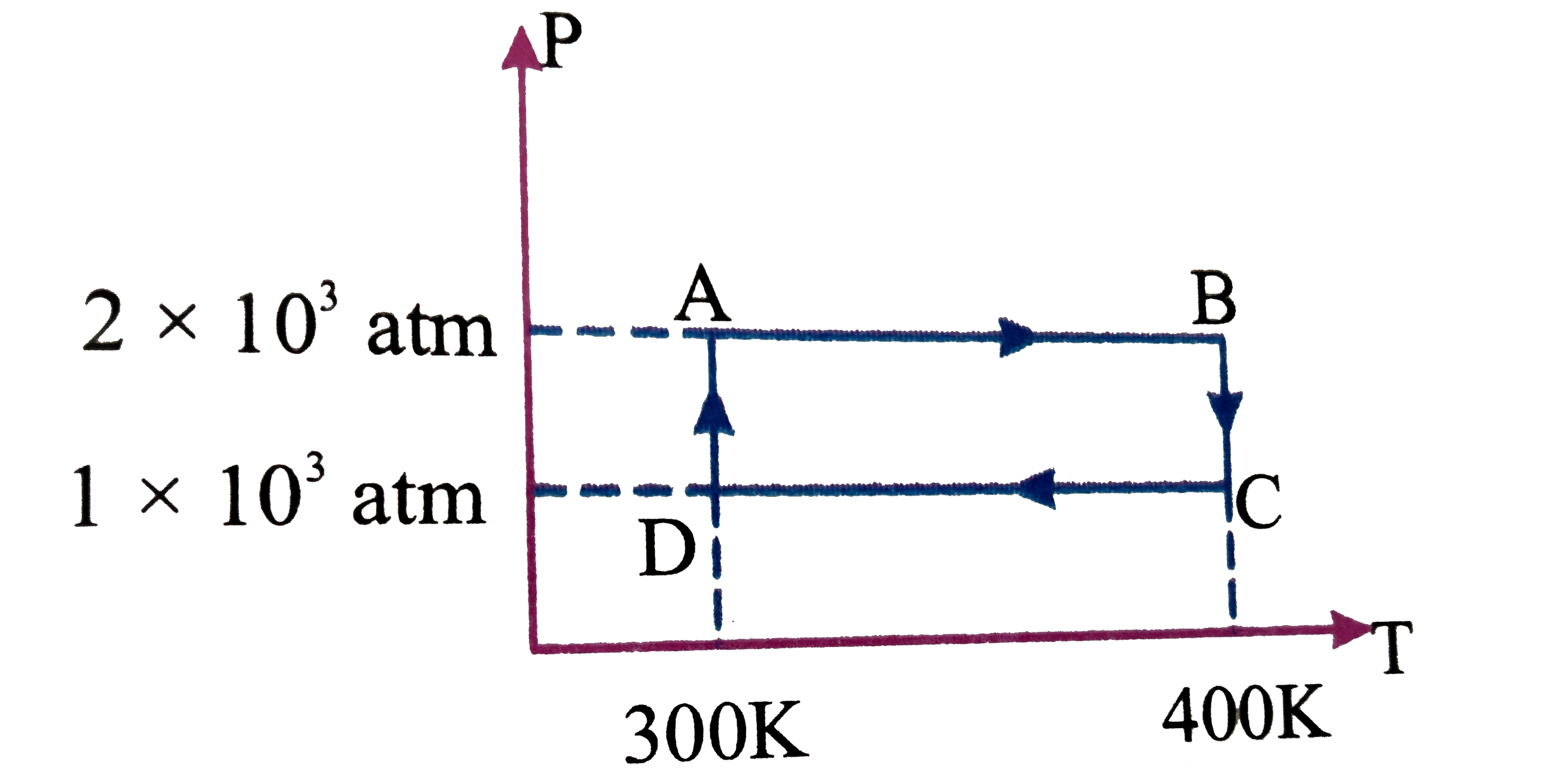
- Two moles of a monatomic ideal gas undergo a cyclic process ABCDA as s...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a process as shown in th...
Text Solution
|
- One mole of an ideal gas undergoes a cyclic change ABCDA as shown in (...
Text Solution
|
- 1//R(R"is universal gas constant") moles of an ideal gas (gamma=1.5) u...
Text Solution
|
- Two moles of Helium gas undergo a reversible cyclic process as shown i...
Text Solution
|
- An ideal gas undergoes cyclic process ABCDA as shown in givend p-V dia...
Text Solution
|
- आदर्श गैस समीकरण में दिखाइए कि सार्वत्रिक गैस नियतांक R का मान 8.31 ज...
Text Solution
|
- An ideal gas undergoes cyclic process ABCDA as shown in given P - V di...
Text Solution
|