Similar Questions
Explore conceptually related problems
Recommended Questions
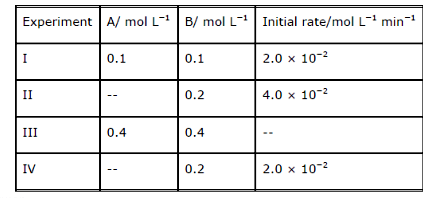
- The reaction between A and B is first order with respect to A and zero...
Text Solution
|
- The reaction between A and B is first order with respect to A and zero...
Text Solution
|
- The reaction between A and B is first order with respect to A and zero...
Text Solution
|
- The reaction between A and B is first order with respect to A and zero...
Text Solution
|
- Fill in the blanks in the following table which treats a reaction of a...
Text Solution
|
- Fill in the balanks in the following table which treats reaction of a ...
Text Solution
|
- A तथा B के बीच अभिक्रिया A के सापेक्ष प्रथम तथा B के सापेक्ष शून्य कोट...
Text Solution
|
- A तथा B के मध्य अभिक्रिया A के प्रति प्रथम तथा के प्रति शून्य कोटि की ...
Text Solution
|
- 3A+2B+CrarrD+E is a first order reaction with respect to A , second or...
Text Solution
|