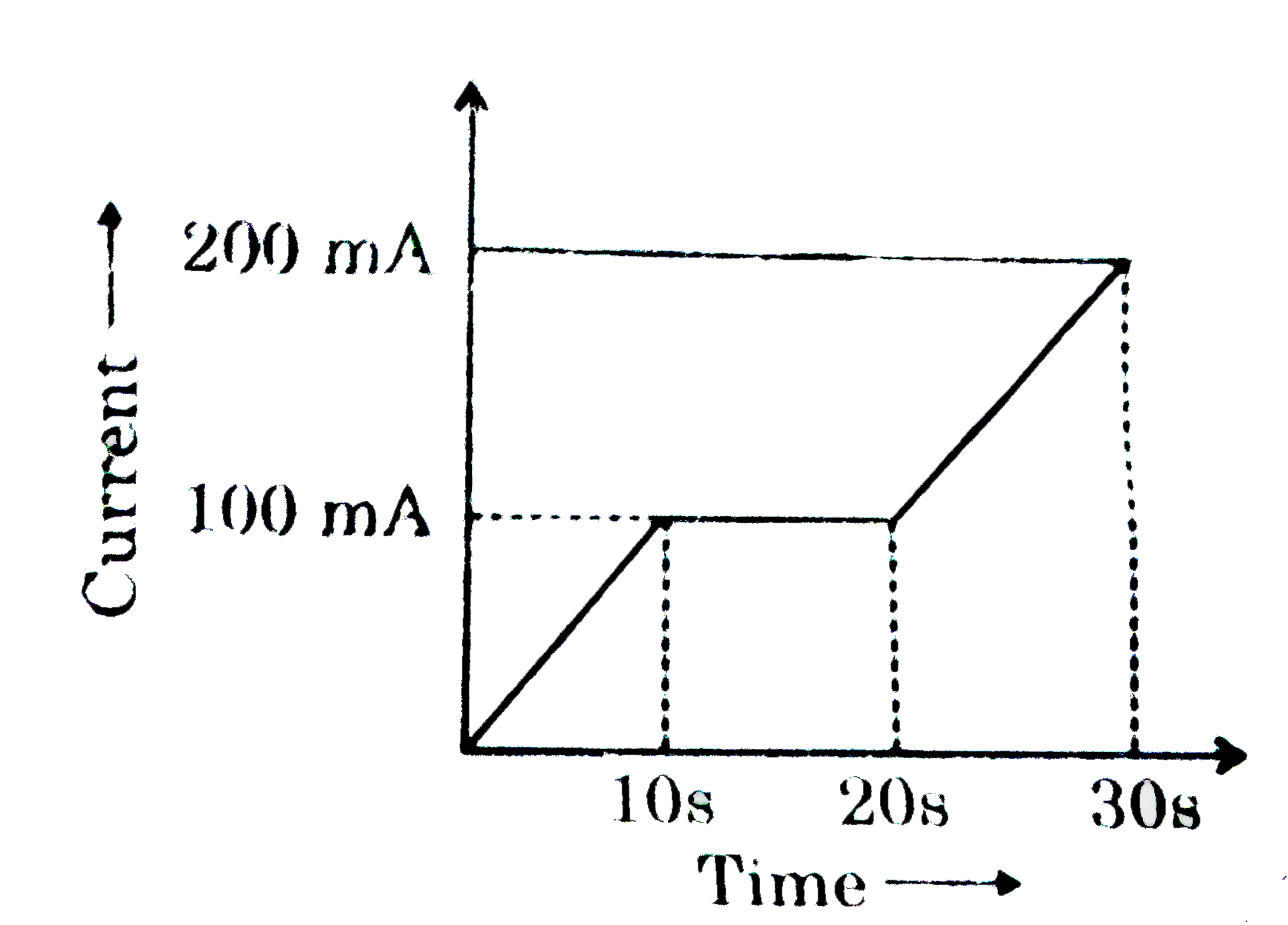Similar Questions
Explore conceptually related problems
Recommended Questions
- In a Cu-voltameter, mass deposited in 30s is m gm. If the time-current...
Text Solution
|
- 0.2864 g of Cu was deposited on passage of a current of 0.5 ampere for...
Text Solution
|
- In a copper voltmeter, mass deposite in 30 seconds is 'm' gram. If the...
Text Solution
|
- For depositing 1 gm of Cu in copper voltameter on passing 2 amperes of...
Text Solution
|
- A current I is passed for a time t through a number of voltameters. If...
Text Solution
|
- E.C.E. of Cu and Ag are 7xx10^(-6) and 1.2xx10^(-6). A certain current...
Text Solution
|
- In a copper voltameter, mass deposited in 6 minutes is m gram. If the ...
Text Solution
|
- In a Cu-voltameter, mass deposited in 30s is m gm. If the time-current...
Text Solution
|
- In a Cu-voltameter, mass deposited in 30 s is m gm. If the time-curren...
Text Solution
|