Similar Questions
Explore conceptually related problems
Recommended Questions
- A resistance coil of resistance r connected to an external battery, is...
Text Solution
|
- In the arrangement shown in Fig. gas is thermally insulated. An ideal ...
Text Solution
|
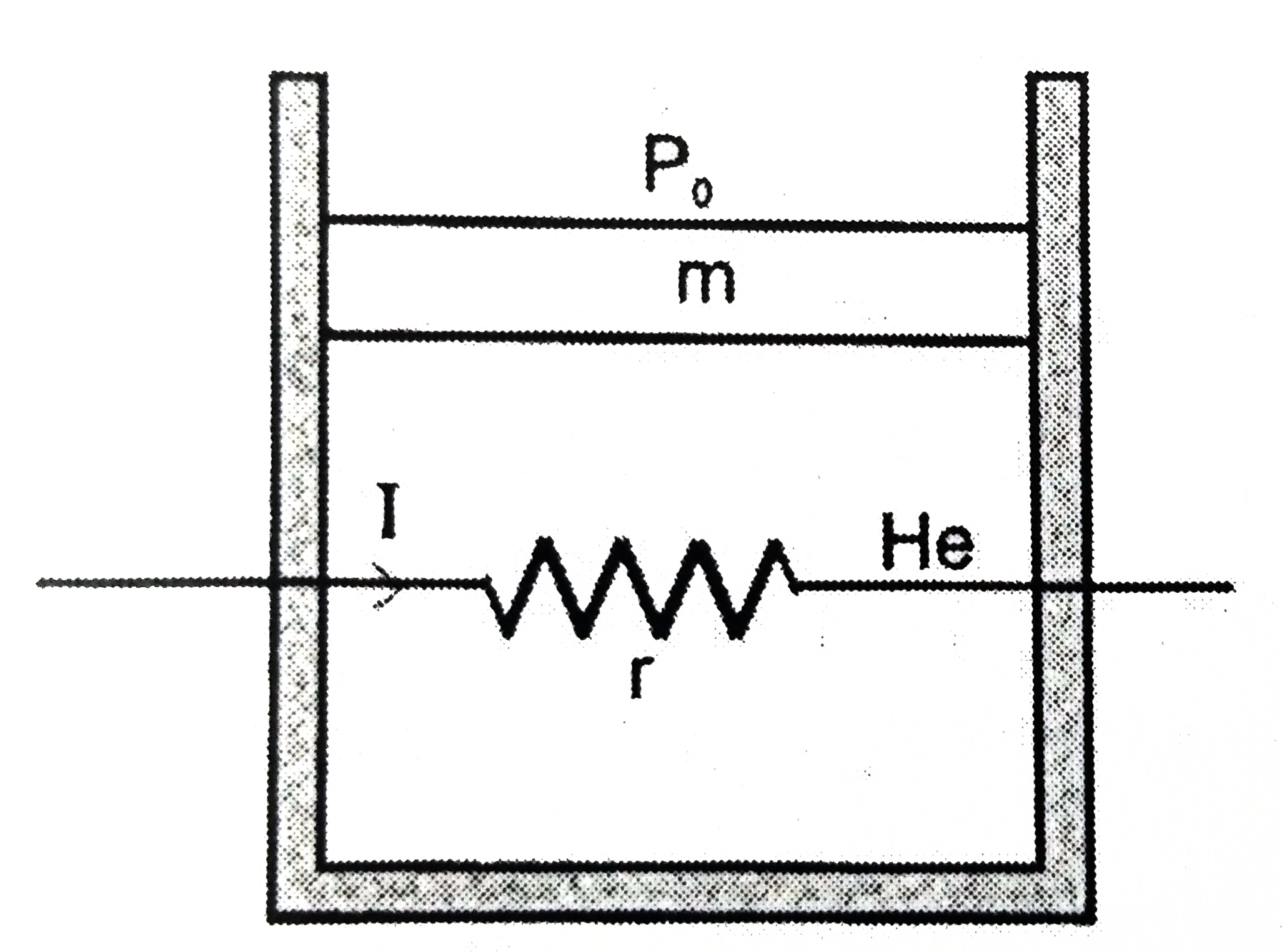
- The air tight and smooth piston of a cylindrical vessel are connected ...
Text Solution
|
- Two coils, 1 & 2, have a mutual inductance = M and resistances R each....
Text Solution
|
- One mole of a perfect gas in a cylinder fitted with a piston has a pre...
Text Solution
|
- A coil having inductance and L and resistance R is connected to a batt...
Text Solution
|
- An adiabatic cylinder of cross section A is fitted with a mass less co...
Text Solution
|
- A resistance coil, connected to an external battery is placed inside a...
Text Solution
|
- One mole of an ideal gas is contained with in a cylinder by a friction...
Text Solution
|