A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -2|70 VideosSOLID STATE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3|22 VideosSOLID STATE
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE -3(VERY SHORT ANSWER QUESTIONS)|5 VideosREVISION EXERCISE
AAKASH SERIES|Exercise COMPLEX COMPOUNDS|47 VideosSOLIDS STATE
AAKASH SERIES|Exercise PRACTICE EXERCISE|63 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-SOLID STATE-OBJECTIVE EXERCISE -1
- The Ca^(2+) and F ions are located in CaF( 2)crystal, respectively at ...
Text Solution
|
- The fraction of the total volume occupied by the atoms present in a si...
Text Solution
|
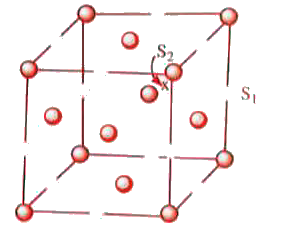
- In the structure given below, the sites S(1) and S(2) represent
Text Solution
|
- The packing fraction for a body centred cube
Text Solution
|
- Density of a crystal is given by :
Text Solution
|
- The percent of void space in a body - centred cubic lattice is :
Text Solution
|
- Calculate the efficiency of the packaing in case of face - centered cu...
Text Solution
|
- Coordination number for Cu is
Text Solution
|
- Which of the following has hcp crystal structure ?
Text Solution
|
- The intermetallic compound LiAg crystallizes in cubic lattice in which...
Text Solution
|
- In a cubic closed packed structure of mixed oxide, the oxide ions are ...
Text Solution
|
- Which of the following has both Schottky and Frenkel defects.
Text Solution
|
- Due to Frenkel defect, the density of the ionic solids
Text Solution
|
- The ratio of Fe^(3+) and Fe^(2+) ions in Fe(0.9)9S(1.0) is
Text Solution
|
- Which of the following has Frenkel defect?
Text Solution
|
- In AgBr, there can occur
Text Solution
|
- If NaCl is doped with 10^(4) mol % of SrCl(2) the concentration of cat...
Text Solution
|
- AB is an ionic solid. If the ratio of ionic radii of A^(+) and B^(+) i...
Text Solution
|
- For an octahedral radius ratio limit is
Text Solution
|
- Which of the following is an example of body centred cube ?
Text Solution
|