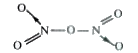Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- What is the covalence of nitrogen in "N"(2)"O"(5) ?
Text Solution
|
- What is the covalence of nitrogen in N2O5?
Text Solution
|
- The maximum covalency of nitrogen is
Text Solution
|
- What is the covalence of nitrogen in N2O5?
Text Solution
|
- Assertion : The covalence of nitrogen in N(2)O(5) is 5 Reason : Nitrog...
Text Solution
|
- What is the covalence of nitrogen in "N"(2)"O"(5) ?
Text Solution
|
- N(2)O(5) में नाइट्रोजन की सहसंयोजकता क्या हैं?
Text Solution
|
- What is the covalence of nitrogen in N(2)O(5) ?
Text Solution
|
- What is the covalence of nitrogen in N(2)O(5) ?
Text Solution
|