Text Solution
Verified by Experts
Topper's Solved these Questions
VII GROUP ELEMENTS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE -1 (Long answer questions)|3 VideosVII GROUP ELEMENTS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE -1(Short answer questions )|8 VideosVIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 5 ( Integer answer type Questions)|6 VideosVIIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE EXERCISE|58 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-VII GROUP ELEMENTS-OBJECTIVE EXERCISE -4
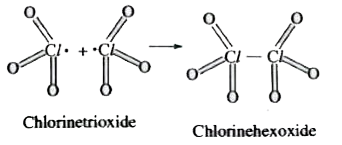
- Chlorine trioxide is paramagnetic, but chlorine hexoxide is diamagneti...
Text Solution
|
- (A): The bond dissociation energy of fluorine is less than bromine. ...
Text Solution
|
- (A): Reaction given below is possible 2KCI + Br2 to Cl(2) + 2KBr (...
Text Solution
|
- (A): Electron affinity of fluorine is lower than that of chlorine (R...
Text Solution
|
- (A): Cl - O bond length decreases from CIO^(-) to ClO(4)^(-) (R): CI...
Text Solution
|
- (A): Bromine does not displace chlorine from its salt solution (R): ...
Text Solution
|
- (A) : lodine is the only halogen that is naturally available in positi...
Text Solution
|
- (A) : Chlorine acts as a bleaching agent in the presence of moisture ...
Text Solution
|
- (A): HBr is stronger acid than HI. (R) : Bromine is more electronega...
Text Solution
|
- (A): Iodine displaces bromine from KBrO3 (R): Iodine is strong oxidi...
Text Solution
|
- (A) OX^(-) on heating in the presence of OH^(-) gives X^(-)andXO(3)^(-...
Text Solution
|
- (A) : Chlorine is gas, bromine is liquid and iodine is solid (R) : I...
Text Solution
|
- (A): Hydrogen iodide is most stable among hydrogen halides (R): Iodi...
Text Solution
|
- (A): Oxidation ability increases from HOCI to HCIO4 (R): Oxidation n...
Text Solution
|
- (A) Aqueous fluoride on electrolysis at inertanode liberates oxygen ...
Text Solution
|
- (A): Fluorine does not form oxyacids (R): Electronegativity of fluor...
Text Solution
|
- (A): lodine appears in violet colour. (R): Iodine absorbsviolet part...
Text Solution
|
- (A) :Among hydrogen halides, hydrogen fluoride is least volatile. (R...
Text Solution
|
- (A) HClO is stronger acid than HBrO (R ) Greater is the electronegat...
Text Solution
|
- (A) : Fluorine is the best oxidising agent (R ) SRP is highest for F...
Text Solution
|
- OF^(-) does not exist , though HOF is known (R ) HOF molecule does n...
Text Solution
|