A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
VII GROUP ELEMENTS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3|12 VideosVII GROUP ELEMENTS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -4|55 VideosVII GROUP ELEMENTS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -1|79 VideosVIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 5 ( Integer answer type Questions)|6 VideosVIIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE EXERCISE|58 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-VII GROUP ELEMENTS-OBJECTIVE EXERCISE -2
- Bond dissociation energies of HF, HCl, HBr follow the order
Text Solution
|
- Which of the following reaction does not takes place.
Text Solution
|
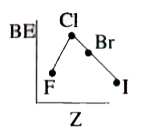
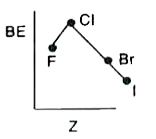
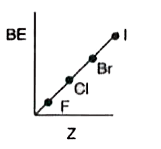
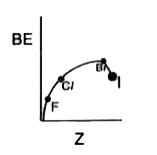
- Which of the following shows variation of BE of halogens
Text Solution
|
- In the reaction 3Br(2) + 6CO(3)^(2-) + 3H(2)OrarrBr^(-) + BrO3 + 6HCO...
Text Solution
|
- Which one of the following ion has the highest value of ionic radius?
Text Solution
|
- Poor conductor of electricity is
Text Solution
|
- No gas is liberated when the following HX is treated with MnO(2)and co...
Text Solution
|
- Which of the following does not form precipitate with AgNO3 ?
Text Solution
|
- Which redical can bring about the highest oxidation state of a transit...
Text Solution
|
- I) Fluorine reacts with cold , dilute alkalies liberating O(2) gas. ...
Text Solution
|
- Which of the following statements is correct about halogens ?
Text Solution
|
- KMnO4 acts as an oxidising agent in alkaline medium, when alkaline KM...
Text Solution
|
- Gaseous HCl is a poor conductor of electricity while its aqueous solut...
Text Solution
|
- Concentrated hydrochloric acid when kept in open air sometimes produce...
Text Solution
|
- Which statement is incorrect
Text Solution
|
- Which of the following is used in the preparation of chlorine ?
Text Solution
|
- Consider the following reaction 6NaOH(("Hot. Conc.")) + 3Cl2 to 5NaC...
Text Solution
|
- A halogen (x) reacts with sulphur gives a compound (y) .(y) reacts wit...
Text Solution
|
- If Cl(2) gas is passed into aqueous solution of KI containing some C C...
Text Solution
|
- NH(3)(excess)+3CltoA+N(2)uarr , the bonds present in compound A is
Text Solution
|