Text Solution
Verified by Experts
Topper's Solved these Questions
COMPLEX COMPOUNDS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE-1 (Short Answer)|6 VideosCOMPLEX COMPOUNDS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE-2 (Short Answer)|2 VideosCO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 5 ( Linked Comprehension type questions Passage : I I:)|3 VideosD & F BLOCK ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET-5 (INTEGER ANSWER TYPE QUESTIONS)|6 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-COMPLEX COMPOUNDS -PRACTICE EXERCISE
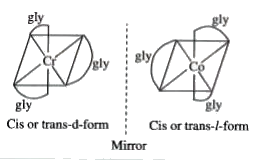
- What type of isomerism is exhibited by the complex (Cr(gly)3) ?
Text Solution
|
- The primary and secondary valencies of the central metal ion in the co...
Text Solution
|
- The complex compound which does not give precipitate with AgNO(3) solu...
Text Solution
|
- the configuration of an element 'X' is 4s^1 3d^10. The wrong statement...
Text Solution
|
- The primary valency of Iron in K(4)[Fe(CN)(6)] is satisfied by
Text Solution
|
- Which of the following is wrong with respect to [Co(NH(3))(5)Cl]Cl(2)
Text Solution
|
- A complex in which central atom carries zero oxidation state is
Text Solution
|
- Oxidation number of metal in the complex [Co(NH(3))(4)Cl(2)]^(+) is
Text Solution
|
- The correct match which is responsible for colour
Text Solution
|
- Give the correct increasing order of electrical conductivity of aqueou...
Text Solution
|
- Coordination number of Cr is 6. A complete entity with C(2)O(4)^(-2), ...
Text Solution
|
- In Aqueous solution meta aluminate ion exists as
Text Solution
|
- How many EDTA molecules are required to make an octahedral complex wit...
Text Solution
|
- Which of the following is neutral molecular complex
Text Solution
|
- The following complex representation violates IUPAC rule
Text Solution
|
- Effective atomic number of central metal ion in [Co(NH3)6]Cl3 is
Text Solution
|
- Complex in which effective atomic number is not equal to atomic number...
Text Solution
|
- IUPAC name of K3[Al(C2O4)3]
Text Solution
|
- The neutral complex, diamminedibromodichloroplatinum(IV) is best repre...
Text Solution
|
- IUPAC name of [Fe(CN)(6)]^(4-) is
Text Solution
|
- The correct match is
Text Solution
|