Text Solution
Verified by Experts
Topper's Solved these Questions
COMPLEX COMPOUNDS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE -1 (SHORT ANSWER QUESTIONS)|5 VideosCOMPLEX COMPOUNDS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE -2 (SHORT ANSWER QUESTIONS)|10 VideosCOMPLEX COMPOUNDS
AAKASH SERIES|Exercise Objective Exercise-4|30 VideosCO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 5 ( Linked Comprehension type questions Passage : I I:)|3 VideosD & F BLOCK ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET-5 (INTEGER ANSWER TYPE QUESTIONS)|6 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-COMPLEX COMPOUNDS -PROBLEM
- Write the formula of the following coordination compounds: (a) Ammin...
Text Solution
|
- Write the formulae for the follow Co-ordination compounds Dichlorido...
Text Solution
|
- Write the formula of the following coordination compounds: (a) Ammin...
Text Solution
|
- Write the formula of the following coordination compound Mercury(I) ...
Text Solution
|
- Arrange the complexes CoCl3. 6NH3, CoCl3. 5NH3, CoCl3. 4NH3 and CoCl2...
Text Solution
|
- When excess ammonia gas is passed through aq CrCl3 solution, complexes...
Text Solution
|
- When excess of silver nitrate solution is added to the aqueous solutio...
Text Solution
|
- The secondary valence of Pt^(4+) is six. Calculate the number of moles...
Text Solution
|
- If [PtCl6]^(x-) follows the Sidgwick rule of stability, what is the o...
Text Solution
|
- If [Fe(CO)x] follows the Sidgwick rule of stability, what is the valu...
Text Solution
|
- The spin only magnetic moment of [MnBr4]^(2-) is 5.9BM. Predict the g...
Text Solution
|
- How the gem stones ruby exhibits red colour and emerald exhibits green...
Text Solution
|
- Anhydrous copper sulphate is colourless, but hydrated copper sulphate ...
Text Solution
|
- Why hexaquamanganese(II)ion contains five unpaired electrons, while th...
Text Solution
|
- How many unpaired electrons present in the square planar [Pt(CN)4]^(2-...
Text Solution
|
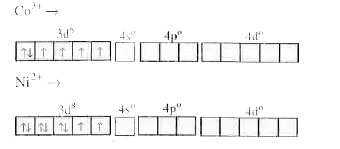
- Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni(NH3)6]^(...
Text Solution
|
- Ag^(+) + NH3 hArr [Ag(NH3)]^(+) , K1=3.5xx10^(-3) , [Ag(NH3)]^(+) +...
Text Solution
|
- CoCl3 . xNH3 exhibits geometrical isomerism. What is the value of x?
Text Solution
|
- Which type of isomerisms are possible with the molecular formula Co(NO...
Text Solution
|
- How is the theory of complexes used to sperate Fe2O3 and Al2O3 ?
Text Solution
|