Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ALCOHOLS, PHENOLS AND ETHERS-ETHERS (OBJECTIVE EXERCISE-4)
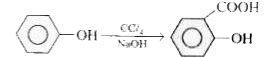
- Phenol is treated with C Cl(4) in presence of NaOH. What happens ?
Text Solution
|
- (A): Alcoholic fermentation involves conversion of sugar into ethanol ...
Text Solution
|
- (A) Sodium can't be used for drying ethyl alcohol. (R) Sodium di...
Text Solution
|
- (A) Ethyl alcohol is soluble in organic solvents (R) Ethyl alcoho...
Text Solution
|
- (A) The boiling point of C2H5OH is less than that of H2O , though the...
Text Solution
|
- (A) Addition of C2H5OH to CH3 MgI gives methane. (R) C2H5OH is mor...
Text Solution
|
- (A): Oxidation of tertiary alcohols requires strong oxidising agent an...
Text Solution
|
- (A) Acetone and propenol-2 are tautomers. (R) Propanone and propen...
Text Solution
|
- (A) Alcohols act as Bronsted - Lowry bases. (R) In alcohols the lo...
Text Solution
|
- (A) Anhydrous ZnCl2 is used in reaction of alcohols with HCI. (R)...
Text Solution
|
- (A) Alcohols can act as nucleophiles (R) Alcohols form salts with...
Text Solution
|
- (A) Tertiary alcohols are more reactive for nucleophilic substitution ...
Text Solution
|
- (A) Reactivity of primary alcohol is more than secondary alcohols towa...
Text Solution
|
- (A) The bond angle C-O-H in alcohol is less than in phenols (R) I...
Text Solution
|
- (A) In ethers, bond angle C-O-C is more than tetrahedral angle (R...
Text Solution
|
- (A) n-propyl alcohol is prepared from propene by the action of B2H6 f...
Text Solution
|
- (A) During acid catalysed esterification, oxygen atom of alcohol is pr...
Text Solution
|
- (A) Lucas reagent is used to distinguish 1^@ , 2^@ and 36@ alcohols ...
Text Solution
|
- (A) Addition of water to (CH3)2 - C = CH - CH3 gives (CH3)2 - COH - C...
Text Solution
|
- (A) 1-propanol & 2-propanol can be distinguished by haloform test ...
Text Solution
|
- (A) Alcohols are acidic in nature but donot react with NaOH solution ...
Text Solution
|