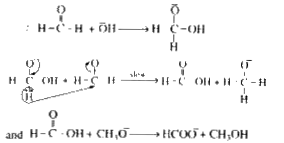Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- In Cannizzaro's reaction, what is the rate determining step ?
Text Solution
|
- What is rate determining step in a complex reaction ?
Text Solution
|
- The rate determining step of a reaction is the step
Text Solution
|
- What is the rate determining step of a reaction ?
Text Solution
|
- कई पदों में सम्पन्न होने वाली अभिक्रिया का वेग निर्धारण पद किसे कहते ह...
Text Solution
|
- In Cannizzaro's reaction 2PhCHOoverset(OH^(-))toPhCH(2)OH+PhCOO^(-) Th...
Text Solution
|
- What is the rate determining step in a multiple step reaction?
Text Solution
|
- In Cannizzaro's reaction, what is the rate determining step ?
Text Solution
|
- In Cannizzaro's reaction, what is the rate determining step ?
Text Solution
|