Text Solution
Verified by Experts
Topper's Solved these Questions
AMINES AND DIAZONIUM SLATS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 1 (AMINES - GENERAL)|13 VideosAMINES AND DIAZONIUM SLATS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 1 (ANILINE)|19 VideosAMINES AND DIAZONIUM SLATS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE -2 (SHORT ANSWER QUESTIONS)|7 VideosAMINES AND AZO COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 6 (Integer answer type Questions)|9 VideosAPPENDICES -REVISION EXERCISE
AAKASH SERIES|Exercise POLYMERS|44 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-AMINES AND DIAZONIUM SLATS-CONVERSIONS
- Accomplish the following conversions. i) Benzoic acid to benzamide ...
Text Solution
|
- Aniline to benzyl alcohol
Text Solution
|
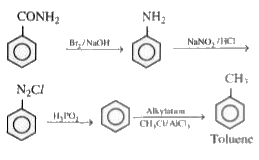
- Benzamide to toluene
Text Solution
|
- Benzylchloride to 2-phenulethanamine
Text Solution
|
- Aniline to sym-tribromofluorobenzene
Text Solution
|
- Chlorobenzene to p-chloroaniline
Text Solution
|
- Ethylchloride to propanamine-1
Text Solution
|
- Ethyl chloride is
Text Solution
|
- How will you convert benzene into N, N-dimethlaniline ?
Text Solution
|
- 1,4-Dichlorobutane to hexane-1,6- diamine
Text Solution
|
- Benzene to p-nitrobenzaldehyde
Text Solution
|
- Benzoic acid to m-nitrobenzylalcohol
Text Solution
|
- Write the structure of the major organic product in each of the follow...
Text Solution
|
- Write the structure of the major organic product in each of the follow...
Text Solution
|
- Write the structure of the major organic product in each of the follow...
Text Solution
|
- Write the structure of the major organic product in each of the follow...
Text Solution
|
- Write the structure of the major organic product in each of the follow...
Text Solution
|
- Write the structure of the major organic product in each of the follow...
Text Solution
|
- Write the structure of the major organic product in each of the follow...
Text Solution
|
- Write the structure of the major organic product in each of the follow...
Text Solution
|