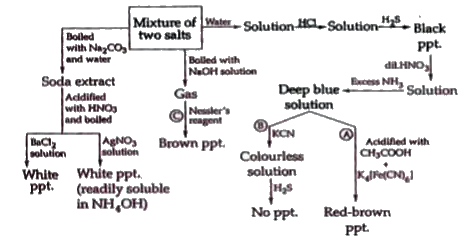
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PRACTICAL CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET-5 (Match the following questions)|3 VideosPRACTICAL CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET-5 (Integer answer Type questions)|6 VideosPRACTICAL CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET-5 (One or more than one answer questions)|8 VideosPOLYMERS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 4|59 VideosPRINCIPLES OF METALLURGY
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE-2 (LONG ANSWER QUESTIONS )|7 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-PRACTICAL CHEMISTRY-PRACTICE SHEET-5 (Linked Comprehension type questions)
- Consider the following sequence of reactions. The white solid A i...
Text Solution
|
- Consider the following sequence of reactions. The change from B t...
Text Solution
|
- Consider the following sequence of reactions. The solution D and ...
Text Solution
|
- The red-brown precipitate formed in step A contains
Text Solution
|
- The colourless solution formed in step B contains
Text Solution
|
- The brown precipitate formed in step C consists of
Text Solution
|