A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- P(4)overset(Ba(OH)(2))underset(Delta)(to) underset("salt")(X) + under...
Text Solution
|
- CH(3)-CH(2)-underset(CH(3))underset(|)overset(CH(3))overset(|)C-unders...
Text Solution
|
- X(C(4)H(9)Br) underset(Delta) overset(alc.KOH)to Yoverset(Br(2)//"CC"l...
Text Solution
|
- X(C(4)H(9)Br) underset(Delta) overset(alc.KOH)to Yoverset(Br(2)//"CC"l...
Text Solution
|
- Graphitie underset(("oxidation"))overset("Strongoxidsing agent")to X("...
Text Solution
|
- P(4)O(6) overset(H(2)O)underset("hot")rarr X +Y uarr then X = and Y =
Text Solution
|
- KOH+NO(2)rarr underset("salt")((A))+underset("salt")((B))overset(Delta...
Text Solution
|
- Ph-underset(OH)underset(|)overset(Ph)overset(|)C-underset(OH)underset(...
Text Solution
|
- (i). Me-underset(OH)underset(|)(C)H-CH(2)-OH underset(Delta)overset(Hl...
Text Solution
|
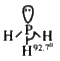 - less soluble than `NH_3` in water as it is weak base
- less soluble than `NH_3` in water as it is weak base