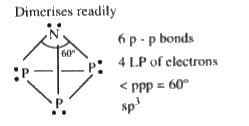A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- White phosphorous (P4) has :
Text Solution
|
- White Phosphorous
Text Solution
|
- In P4 O10 , how many oxygen atoms are bonded to each phosphorous atoms...
Text Solution
|
- White phosphorus (P4) has
Text Solution
|
- (A) : White phosphorous is less reactive than red phosphorous (R) : Wh...
Text Solution
|
- Assertion: Red phophorus is less volatile then white phosphorus. Rea...
Text Solution
|
- In the preparation red phosphorous from white phosphorous
Text Solution
|
- श्वेत फॉस्फोरस वाष्प अवस्था में ………….. सघंटन रखता है ।
Text Solution
|
- White phosphorous has which of the following structure?
Text Solution
|