Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- When ammonia is heated with cupricoxide . How many electrons will each...
Text Solution
|
- How many molecules of ammonia are required to form 8 molecules of urea
Text Solution
|
- When ammonia is heated with cupric oxide, a molecule of ammonia will
Text Solution
|
- How many electrons and ATP are required for synthesis of 4 molecules o...
Text Solution
|
- How many molecules of hydrogen is required to produce 4 moles of ammon...
Text Solution
|
- What is the mass of 1millimol ammonia? How many molecules of ammonia a...
Text Solution
|
- How many gram-molecules and how many grams of ammonia are there in 100...
Text Solution
|
- When ammonia is heated with cupricoxide . How many electrons will each...
Text Solution
|
- NTP -তে 5.6 L অ্যামোনিয়ার মধ্যে কটি অণু আছে ?
Text Solution
|
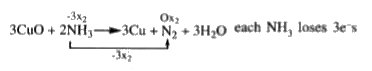 each `NH_3` loses `3e^(-)s`
each `NH_3` loses `3e^(-)s`