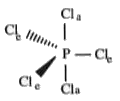
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- PCl(5) in solid state exists as PCl(4)^(+) and PCl(6)^(-) and also in ...
Text Solution
|
- Asserartion:PCl(5) is covalent in gaseous and liquide states but ionic...
Text Solution
|
- In a polar solvent, PCl(5) undergoes an ionization reaction as : " ...
Text Solution
|
- Assertion : PCl(5) is covalent in gaseous and liquid state but ionic i...
Text Solution
|
- In solid PCl(5) exist as [PCl(4)]^(+) [PCl(6)]^(-) . The hybridisation...
Text Solution
|
- (a) In a polar solvenbt , PCl(5) undergoes an ionization reaction as f...
Text Solution
|
- In a certain polar solvent, PCl(5) undergoes ioonisation as follows: 2...
Text Solution
|
- In a certain polar solvent, PCl(5) undergoes ioonisation as follows: 2...
Text Solution
|
- In the gas phase PCl(5) exists as individual molecules but in the soli...
Text Solution
|