Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The total number of lone pairs in PH3 molecule is
Text Solution
|
- The total number of lone pairs in a chlorine molecule is
Text Solution
|
- Numbers of lone pairs present in ozone molecule is :-
Text Solution
|
- The number of lone pairs in hydrogen fluoride molecule are
Text Solution
|
- Number of lone pairs of electrons in P(4) molecule = …….. .
Text Solution
|
- The molecule which contains maximum number of lone Pair is
Text Solution
|
- The total number of lone pair electron that found in F2 molecules is
Text Solution
|
- The number of lone pair of electrons in water molecule is ……………………
Text Solution
|
- The total number of bond pairs and lone pairs around oxygen Lewis stru...
Text Solution
|
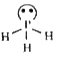 one lone pair
one lone pair