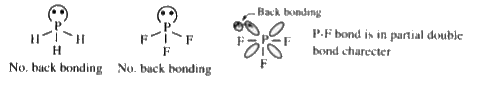A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The bond angle in PH3 is less than bond angle in PF3 This is due to
Text Solution
|
- The bond angle in PH3 is :
Text Solution
|
- The bond angle in PH3 is close to
Text Solution
|
- In NH(3), bond angle is less than tetrahedral bond angle due to .
Text Solution
|
- NF3অণুতে F-N-F বন্ধন-কোণের মান NH3 অণুর H-N-H বন্ধন-কোণের মান অপেক্ষা...
Text Solution
|
- Bond angle in PH4^(+) is higher than that in PH3 . Why?
Text Solution
|
- NH3 में बंध कोण चतुष्फलकीय बंध कोण की अपेक्षा कम होता है इसका कारण है
Text Solution
|
- The bond angle in PH3 is less than bond angle in PF3 This is due to
Text Solution
|
- Why bond angle of Phosphine (PH3) is less than Ammonia (NH3) ?
Text Solution
|