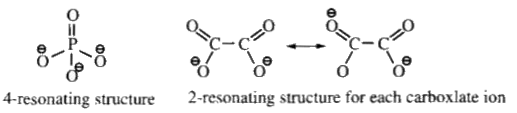
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the ionic reaction : HC(2)O(4)^(-) +PO(4)^(-3) hArr HPO(4)^(2...
Text Solution
|
- Calculate the pH of 0.05 M KHC(8)H(4)O(4) H(2)C(8)H(4)O(4) + H(2)O hAr...
Text Solution
|
- H(3)PO(4)hArr H^(o+) +H(2)PO(4)^(Theta), K(a(1)) : H(2)PO(4)^(Theta) h...
Text Solution
|
- The reaction H(3)PO(4)+Ca(OH)(2)rarrCa(HPO(4))(2)+2H(2)O Which stateme...
Text Solution
|
- In the following reaction HC(2)O(4)^(-)(aq)+PO(4)^(3-)(aq)hArrHPO(4)^(...
Text Solution
|
- For H(3)PO(4), H(3)PO(4) hArr H(2)PO(4)^(-) + H^(+) (K(1)) H(2)PO(4...
Text Solution
|
- Consider the equilibrium reactions, H(3)PO(4)overset(K(1))(hArr ) H^(+...
Text Solution
|
- In the following reaction HC(2)O(4)^(-) + PO(4)^(--) hArr HPO(4)^(--) ...
Text Solution
|
- The two Bronsted bases in the reaction are HC(2)O(4)^(-)+PO(4)^(3-) to...
Text Solution
|