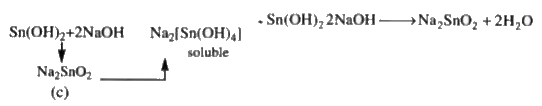A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- An inorganic compound (A) that is transparant like glass is strong red...
Text Solution
|
- An inorganic compound (A) loses its water of crystallisation on heatin...
Text Solution
|
- (A) (colourless solution ) gives white ppt (B) with NaOH solution but ...
Text Solution
|
- An inorganic compound (A) is white solid and exist as dimer. (A) get...
Text Solution
|
- An inorganic compound (A) is white solid and exist as dimer. (A) get...
Text Solution
|
- An inorganic compound (A) is white solid and exist as dimer. (A) get...
Text Solution
|
- An inorganic compound (A) , transparent like glass is a strong reducin...
Text Solution
|
- The compound which gives white ppt. with aqueous AgNO3 and a green fla...
Text Solution
|
- Transparent glassy solid strongly reducing, purple of cassius reduces ...
Text Solution
|