Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- How many number of lone pairs are present in central atom of C//f(3) ?
Text Solution
|
- The number of lone pairs of electrons present on the central atom of C...
Text Solution
|
- The number of lone pairs of electrons present in central atom of CIF3 ...
Text Solution
|
- The number of lone pair of electrons in central atom of CIF5
Text Solution
|
- The ion with maximum number of lone pairs on the central atom is-
Text Solution
|
- How many bonding pairs and lone pairs surround the central atom in the...
Text Solution
|
- In which of the following molecules maximum number of lone pairs is pr...
Text Solution
|
- Only one lone pair is present on the central atom of
Text Solution
|
- The number of lone pairs present on the central atom of XeF2, XeF, and...
Text Solution
|
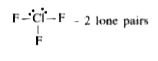 lone pairs
lone pairs