Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The basicity of HClO(4) is 'X'. What is the value of 'X' ?
Text Solution
|
- Assertion: HClO(4) is a stronger acid than HClO(3). Reason: Oxidatio...
Text Solution
|
- Assertion: HClO(4) is a stronger acid than HClO(3) . Reason: Oxidation...
Text Solution
|
- The correct decreasing order of the acidic strength of HClO, HClO(2), ...
Text Solution
|
- (A) HClO(4) is stronger acid than HClO(3). (R ) Oxidation state of C...
Text Solution
|
- Out of HClO(3) " and " HClO(4) which has lower pk(a) value and why ?
Text Solution
|
- The correct statements about the oxoacids HClO(4) " and " HClO(3) is (...
Text Solution
|
- What is the oxidation state of Cl in HClO(4) ?
Text Solution
|
- The correct statement (s) regarding, (i) HClO. (ii) HClO(2), (iii) HCl...
Text Solution
|
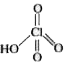 NU repleacable .H. is .I.
NU repleacable .H. is .I.