Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- How many number of hybrid orbitals of Chorine are present in ClO(4)^(-...
Text Solution
|
- How many number of d-electrons are present in[Co(NH(3))(5)CO(3)]ClO(4)...
Text Solution
|
- The type of hybrid orbitals used by chlorine atom in ClO(2)^(-) is :
Text Solution
|
- How many hybrid orbitals are used for bond formation in SF(4) ?
Text Solution
|
- The type of hybrid orbitals used by chlorine atom in ClO(3)^(-) is
Text Solution
|
- sp^(3) संकरित कक्षकों (ऑर्बिटलों ) में कितना s तथा p लक्षण उपस्थित...
Text Solution
|
- How many 'sp' hybrid orbital are there in allene C(3)H(4) ?
Text Solution
|
- Number of hybrid orbitals present in a molecule of propene are
Text Solution
|
- The type of hybrid orbitals used by the chlorine atom in ClO(2)^(-) io...
Text Solution
|
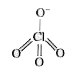 In `ClO_(4)^(-)` hybridisation of `Cl_(2)` is `sp^(3)` (i.e., 4 orbitals )
In `ClO_(4)^(-)` hybridisation of `Cl_(2)` is `sp^(3)` (i.e., 4 orbitals )