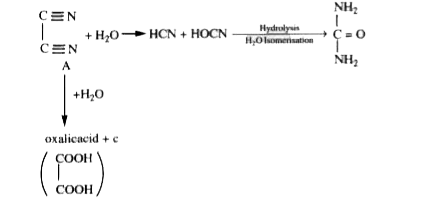A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In was the most important one type of Halogen compound which is the fi...
Text Solution
|
- Identify A and B in the reaction given below "Ethane nitrile"underse...
Text Solution
|
- "Glucose"underset(A)underset(darr)to "Pyruvic acid" underset(B)underse...
Text Solution
|
- "Glucose"underset(A)underset(darr)to "Pyruvic acid" underset(B)underse...
Text Solution
|
- CH(3)C C l(2)CH(3)underset("Hydrolysis")overset(NaOH)(rarr)P overset(-...
Text Solution
|
- Compound A on reaction with HCN andon further hydrolysis gives C(2)H(5...
Text Solution
|
- Compound A on reaction with HCN andon further hydrolysis gives C(2)...
Text Solution
|
- Under ambient condition , the total number of gases released products ...
Text Solution
|
- Glucose overset(HCN)rarr (X) overset("hydrolysis")rarr (Y) underset(De...
Text Solution
|