Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Oxidation number of Cl in Cl(2) O(6) compound.
Text Solution
|
- Among [Ni(CO)(4)], [NiCl(4)]^(2-), [Co (NH(3))(4) Cl(2)] Cl, Na(3) [Co...
Text Solution
|
- What is the average oxidation number of tungesten in the ion , W(6)O(6...
Text Solution
|
- यौगिक C(6)H(6)Cl(2) के संभावित समावयवियो की संख्या है :
Text Solution
|
- Cl(2)O(3), Cl(2)O तथा Cl(2)O(5) ऑक्साइडों को उनकी अम्लीयता के बढ़ते क्र...
Text Solution
|
- In the coordination compound [Co(en)(2)Cl(2)]Cl (en=ethylenediamine), ...
Text Solution
|
- Among [Ni(CO)(4)], [NiCl(4)]^(2-), [Co(NH(3))(4)Cl(2)]Cl, Na(3)[CoF(6)...
Text Solution
|
- Cl(2)O(6)+NaOH to ?
Text Solution
|
- Among [Ni(CO)(4)], [NiCl(4)]^(2-), [Co (NH(3))(4) Cl(2)] Cl, Na(3) [C...
Text Solution
|
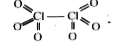 `2x + ( -2)6 = 0, 2x = 12, x = 6` o.n OF Cl in `Cl_(2) O_(6) ` is ` = + 6`
`2x + ( -2)6 = 0, 2x = 12, x = 6` o.n OF Cl in `Cl_(2) O_(6) ` is ` = + 6`