Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The bond order of Cl-O in ClO(4)^(-) is (x)/( y ) then x-y=?
Text Solution
|
- Cl 2 on reaction with hot & conc. NaOH gives two chlorine having ...
Text Solution
|
- Calculate the bond order of Cl-O bond in ClO(4)^(-).
Text Solution
|
- The correct order of Cl–O bond order is :
Text Solution
|
- The correct order of o^(-) bond lengths in ClO^(-), ClO(2)^(-), ClO(3)...
Text Solution
|
- The correct order of o^(-) bond lengths in ClO^(-), ClO(2)^(-), ClO(3)...
Text Solution
|
- Chlorine react with hot and concentrated NaOH and produces compound (X...
Text Solution
|
- The order of Cl - O bond energy in ClO^(-), ClO(2)^(-), ClO(3)^(-), CI...
Text Solution
|
- (A): Cl - O bond length decreases from CIO^(-) to ClO(4)^(-) (R): CI-O...
Text Solution
|
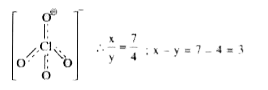 `:. (x)/( y ) = ( 7 )/( 4) , x - y = 7-4 = 3`
`:. (x)/( y ) = ( 7 )/( 4) , x - y = 7-4 = 3`